"Making Difficult-to-Make Molecules: Photochemistry as an Enabling Tool"
- Date(s)
- January 20, 2021
- Location
- School of Chemistry and Chemical Engineering - ONLINE seminar
- Time
- 14:00 - 15:00
Converting simple starting materials into complex products using only a light source (synthetic photochemistry) is especially attractive to organic chemists, particularly from the point of view of green chemistry: waste is minimized, and light is readily available. In addition, photochemical routes often allow efficient access to complex frameworks (particularly to strained molecules and intermediates) that cannot be generated using ground-state chemistry.
In this seminar, Dr Coote's recent work on the synthesis and applications of bicyclic 1,2-diazetidines2 will be presented. Bicycles2 can be obtained in high yields from 1,2-dihydropyridazines1 simply upon irradiation at 350 nm, and are versatile synthetic intermediates that can be converted into a variety of different derivatives, including substituted 1,2-diazetidines, cyclobutanes, cyclobutenes and dienes (Figure 1).1,2,3 In addition, related work on similar systems will be discussed, including ongoing unpublished work.
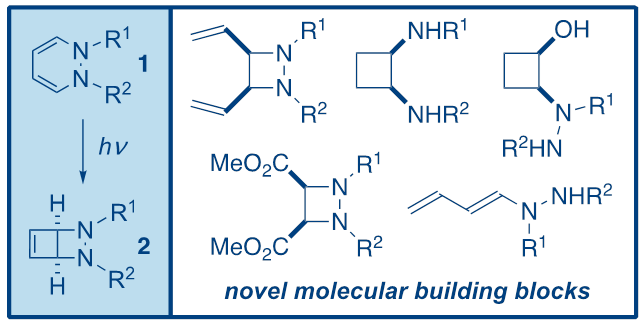
Figure 1.
References
1. Britten, T. K.; Kemmitt, P. D.; Halcovitch, N. R.; Coote, S. C. Org. Lett. 2019, 22, 9232
2. Britten, T. K.; Akien, G. R.; Kemmitt, P. D.; Halcovitch, N. R.; Coote, S. C. Tetrahedron Lett. 2019, 60, 1497
3. Britten, T. K.; Kemmitt, P. D.; Halcovitch, N. R.; Coote, S. C. Synlett 2020, 31, 459

