Dynamic Regulatory Alignment and the Protocol on Ireland/Northern Ireland - One Year
Lisa Claire Whitten
January 2022
Download a copy of this Explainer here.
Introduction
Northern Ireland occupies a unique position in the relationship between the United Kingdom (UK) and the European Union (EU). The Protocol on Ireland/Northern Ireland provides that aspects of EU law continue to apply in Northern Ireland despite it having left the EU along with the rest of the UK on 31 January 2020.
Under the terms of the Protocol, Northern Ireland remains part of the UK customs territory. However, the EU customs code continues to apply in respect of Northern Ireland as do specified EU acts that regulate the free movement of goods, VAT and excise, state aid and electricity markets. New EU acts that fall within the scope of the Protocol may also be added to those that apply in Northern Ireland.
Moreover, the Protocol requires that amendments or replacements to these acts apply in Northern Ireland. Such dynamic regulatory alignment is necessary to maintain the free movement of goods on the island of Ireland. However, this has proved politically controversial, not least because it involves EU acts applying in Northern Ireland in which neither the UK nor Northern Ireland has had a direct role in adopting.
Focusing on the policy and legal implications, this explainer reviews the first year of ‘dynamic regulatory alignment’ with the EU in post-Brexit Northern Ireland. In doing so, it builds on a previous review carried out after the first six months of Northern Ireland’s ‘dynamic regulatory alignment’ with the EU. The extent of change after one year of the Protocol does not differ much from that presented after six months of its implementation; this is itself an important finding. The absence of significant change in the second half of 2021 reflects the slow pace of the EU legislative process and relative stability of the specific set of EU acts that continue to apply in the UK in respect of Northern Ireland.
Given the slow pace of change, the policy impacts of ‘dynamic regulatory alignment’ for Northern Ireland, post-Brexit, are not as extensive as they could be. This is not to say dynamic regulatory alignment does not raise challenges in respect of democratic accountability and capacity for legislative scrutiny. Following a review of the first year of implementation, the conclusion to this explainer returns to these dual challenges.
1. The new dynamism of Northern Ireland
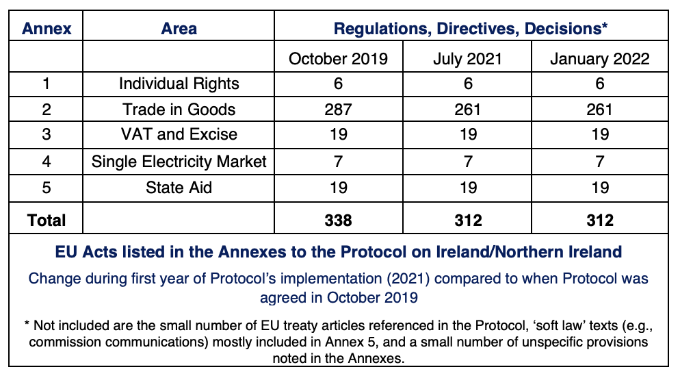
Under Article 13(3) of the Protocol, EU acts listed in Annexes to the Protocol apply ‘as amended or replaced’ to the UK in respect of Northern Ireland. When the Protocol and its Annexes were agreed by UK and EU negotiators as part of the UK-EU Withdrawal Agreement in October 2019, 338 acts were listed. Under the Protocol, additions can be made, and acts can also be deleted.
This means that, under the Protocol, Northern Ireland is in a position of ‘dynamic alignment’ with a specified but potentially evolving selection of the EU ‘acquis’, the body of legal and other agreed obligations and commitments that apply to, and in, EU member states.
In implementing the Protocol, therefore, the UK must keep Northern Ireland aligned with any changes made to the EU acts included in the scope of the Protocol.
One year into the full implementation of the Protocol, how has the body of EU law that applies to, and in, Northern Ireland changed?
Like many issues in the world of Brexit, the answer is not simple. Several different types of change have taken place. They fall into three broad categories:
- additions to and deletions from the Annexes to the Protocol;
- repeal, replacement, and expiry of applicable EU law; and
- changes to EU legislation implementing applicable EU law.
2. Additions to and deletions from the Annexes to the Protocol
The first category of change concerns the specific EU acts that apply in Northern Ireland under the Protocol. Through the Joint Committee set up to oversee the implementation of the UK-EU Withdrawal Agreement, the UK and the EU can, by agreement, add new EU acts to the Annexes to the Protocol. The Joint Committee can also remove acts listed.
Before the end of the transition period, in December 2020, the UK and EU agreed to add eight EU acts to Annex 2 of the Protocol. It also agreed to remove two EU acts listed in the same Annex.
Of the eight acts added, five related to legislation that the Joint Committee decided, following review, should have been included in the original text of Annex 2. The five acts that were added are:
- rules for monitoring trade between the EU and third countries in drug precursors (Council Regulation (EC) 111/2005);
- use of indications or marks to identify the lot – or batch – to which food products belong (Directive 2011/401/EU);
- rules on the marketing of fodder plant seed (Council Directive 66/401/EEC);
- rules on the marketing of propagating material of ornamental plants (Council Directive 98/56/EC); and
- rules on the marketing of vegetable propagating and planting material other than seed (Council Directive 2008/72/EC).
The three other additions were new EU acts adopted since the content of the Protocol had initially been agreed in November 2018. The Joint Committee decided that the following three acts fell within the scope of the Protocol, so added these to Annex 2:
- bilateral safeguard clauses and other mechanisms for the temporary withdrawal of preferences in certain EU trade agreements with third countries (Regulation (EU) 2019/287);
- measures to reduce the impact of certain plastic products on the environment (Directive (EU) 2019/904); and
- and measures to control the introduction and import of cultural goods (Regulation (EU) 2019/880).
The two acts that were removed by the Joint Committee concerned CO2 emissions standards for passenger cars (Regulation (EC) 443/2009) and light-duty commercial vehicles (Regulation (EU) 510/2011). Their original inclusion was deemed unnecessary.
Taking these changes into account, when the Protocol entered into force on 1 January 2021 following the end of the transition period, 344 EU acts were listed in its Annexes. No further additions or deletions have since been made.
3. Repeal, replacement, and expiry of applicable EU Law
The second category of change covers the repeal, replacement, and expiry of EU acts – regulations, directives, and decisions – listed in the Annexes to the Protocol. Changes in this category are the result of normal EU legislative processes and follow from the provision in Article 13(3) of the Protocol stating that relevant EU acts apply as ‘amended or replaced’ to and in Northern Ireland.
Of the 338 EU legal acts originally listed in the Annexes, 49 had been repealed as of 1 January 2022. Not all were directly replaced by a new piece of EU legislation, however. This is because several relevant changes consolidated provisions previously spread over numerous pieces of (now repealed) legislation, into one or two new, more comprehensive, acts.
Of the 49 repealed acts, 19 have been replaced. In most instances, this dynamic alignment concerns changes to pieces of EU legislation that had been adopted prior to the UK’s withdrawal from the EU on 31 January 2020. Of the 19 replacement acts, only four were adopted after the UK left the EU.
In terms of coverage, 23 of the 49 repealed acts concerned controls on animal health and were replaced by two new pieces of legislation: Regulation (EU) 2016/429 and Commission Delegated Regulation (EU) 2020/687. The former is known as the ‘Animal Health Law’ and the latter is a related, supplementary act. Together these two new acts incorporate, and update pre-existing provisions set out in the 23 repealed acts.
The changes laid down in the Animal Health Law were agreed in March 2016, before the UK’s EU referendum and therefore with the UK taking full part in their adoption. The original text included transitional measures and allowed for the repeal of the earlier acts to take effect in April 2021.
As a supplement to the 2016 Regulation, the Commission Delegated Regulation (EU) 2020/687 sets out measures to prevent and control the spread of certain diseases. The relevant diseases were listed in the 2016 regulation but required more specific provisions.
In a similar way, seven of the other repealed acts concerned EU rules on official controls and checks on food and feed, animal health and welfare standards, plant health and plant protection. These were replaced by a single overarching EU act: Regulation (EU) 2017/625, known as the ‘Official Controls Regulation’. It incorporates and updates pre-existing provisions in the repealed acts. It was agreed in April 2017, shortly after the UK triggered Article 50 announcing its intended withdrawal from the EU, and so with the UK participating in the regulation’s adoption. The two regulations included transitional measures and allowed for the repeal of the earlier acts to take effect in December 2019.
Also repealed were two directives – Council Directive 93/42/EEC and Council Directive 90/385/EEC – concerning the production of and trade in medical devices. This had been provided for elsewhere in Regulation (EU) 2017/745 which was already listed in Annex 2 to the Protocol, so the repealed directives were not replaced directly.
In addition, two regulations concerning requirements for the use of statistics on (respectively) trade in goods between EU member states and with non-EU countries – Regulation (EC) No 638/2004 and Regulation (EC) No 471/2009 – were repealed and replaced by Regulation (EU) 2019/2152 on European business statistics that incorporates and updates requirements from the earlier acts. The new regulation was agreed in November 2019, when the UK was still an EU member state; it also included transitional measures for the scheduled repeal of earlier acts to take effect at the end of 2021.
A further 15 repealed regulations and directives originally listed in the Protocol have been directly replaced by new acts. Of these, four concern the regulation of electricity markets and energy supplies (Directive 2009/72/EC, Regulation (EC) 714/2009, Regulation (EC) 713/2009 and Directive 2005/89/EC) and were originally listed in Annex 4, supplementing Article 9 of the Protocol which makes provision for the continued operation of the Single Electricity Market on the island of Ireland.
The four acts originally listed in Annex 4 have been replaced by four updated acts (Directive (EU) 2019/944, Regulation (EU) 2019/943, Regulation (EU) 2019/942 and Regulation (EU) 2019/941 respectively) between July 2019 and December 2020. The replacement acts cover the same policy areas and implement changes agreed in June 2019 – again while the UK was still a member state of the EU.
The remaining eleven acts that have been repealed and replaced directly concern:
- the approval and market surveillance of motor vehicles and related products (Directive 2007/46/EC) replaced by Regulation (EU) 2018/858 adopted in June 2018;
- controls on cash entering or leaving the EU (Regulation (EC) 1889/2005) replaced by Regulation (EU) 2018/1672 adopted in November 2018;
- controls on trade in goods that could be used in capital punishment or torture (Council Regulation (EC) 1236/2005) replaced by Regulation (EU) 2019/125 adopted in January 2019;
- the mutual recognition of goods between member states (Regulation (EC) 764/2008) replaced by Regulation (EU) 2019/515 adopted in March 2019;
- controls on persistent organic pollutants (Regulation (EC)8 50/2004) replaced by Regulation (EU) 2019/1021 adopted in June 2019;
- the marketing and use of explosives precursors (Regulation (EU) 98/2013) replaced by Regulation (EU) 2019/1148 adopted in July 2019;
- provisions for the conservation of fisheries and marine ecosystems (Council Regulation (EC) 850/98) replaced by Regulation (EU) 2019/1241 adopted in July 2019;
- provisions for computerising the movement and surveillance of exercisable goods (Decision 1152/2003/EC) replaced by Decision (EU) 2020/263 adopted in February 2020;
- rules on the labelling of tyres (Regulation (EC) 1222/2009) replaced by Regulation 2020/740 adopted in June 2020;
- controls on the acquisition and possession of weapons (Council Directive 91/447/EEC) replaced by Directive (EU) 2021/555 adopted in April 2021; and
-
the EU regime for the control of exports, transfer, brokering and transit of dual-use items (Regulation (EC) 428/2009) repealed by Regulation (EU) 2021/821 adopted in May 2021 but with provision for the continued application of authorisations made under the earlier act and before 9 September 2021.
Overall, then, of the 19 new acts that have replaced the 49 repealed acts, only four have been adopted by the EU since the UK’s withdrawal on 31 January 2020. Of these, just two were adopted by the EU during 2021.
In addition to the repealed acts, two acts originally listed in the Annexes expired after the UK withdrew from the EU. These concerned the regulation of imports from third countries affected by the Chernobyl disaster (Council Regulation (EC) 733/2008) and temporary trade measures for goods originating in Ukraine (Regulation (EU) 2017/1566).
Taking all of these changes into account alongside those agreed by the Joint Committee in December 2020, the number of EU acts that apply in post-Brexit Northern Ireland has actually decreased since the Protocol entered into force. As of 1 January 2022, there are now 312 EU regulations, directives and decisions that apply; 26 less than when the Protocol was first agreed in October 2019.
4. Changes to EU legislation implementing applicable EU law
The third category of change relates to legislation that implements the regulations, directives and decisions listed in the Annexes to the Protocol. As in the second category – repeal, replacement, and expiry – this type of change is the result of normal EU legislative processes. It also follows from Article 13(3) of the Protocol.
To understand the significance (or otherwise) of this third category it is helpful to first explain what EU implementing legislation is and why it exists.
Often EU directives, regulations, and decisions, such as those listed in the Annexes to the Protocol, are written in quite general terms. They are, after all, designed to apply in 27 member states. This means, however, that the original ‘parent’ act does not always set out in sufficient detail all the procedures, processes or requirements that may be necessary to implement its provisions.
So, to avoid unhelpful ambiguity or unconstructive variation in the way a new law is implemented, EU acts often provide for ‘implementing legislation’ to be adopted. Such legislation is within the scope of a given ‘parent’ legal act and sets out the rules and procedures for its operationalization and is adopted after the original parent act has passed.
EU implementing legislation – including that relevant under the Protocol – is regularly adopted by either the Commission or the Council. Each year over 1000 pieces of implementing legislation are adopted. This is a high figure, one that reflects the extent to which implementing legislation is used as a tool in the application of EU law and policy. It is important to note, however, that most implementing acts concern technical and specific issues, and they always remain within the scope of the original ‘parent’ act.
By way of demonstration, on 6 December 2021, Commission Implementing Decision (EU) 2021/2184 and Commission Implementing Decision (EU) 2021/2185 were made under Article 55(1) of Regulation (EU) 528/2012 concerning the making available on the market and use of biocidal products and which, under Article 5 and Annex 2 of the Protocol, applies in Northern Ireland under the Protocol. The purpose of these Commission implementing decisions was to extend permission for the use of certain hand sanitizer products that use propan-2-ol and sodium hypochlorite in Northern Ireland. Under the ‘parent’ regulation, the companies producing these hand sanitizers are required to go through an authorisation process because of the chemicals involved. Prior to the COVID-19 pandemic, however, few hand disinfectant products were authorised and in use in the UK. The huge spike in demand for such products since the pandemic began could not therefore be met by existing authorised producers alone. This situation led in 20 November 2020 to the UK taking a decision under Article 55(1) of Regulation (EU) 528/2012 that allows ‘competent authorities’ to derogate, temporarily, from the normal requirements for authorisation of biocidal products in exceptional circumstances. In this case, the UK Health and Safety Executive – acting on behalf of the Health and Safety Executive for Northern Ireland – decided in the light of the pandemic to permit the use of hand sanitizer products in Northern Ireland not yet authorised. Article 55(1) allows competent authorities to permit an initial derogation for a period up to 180 days, but this can be extended if a reasoned request is made by the relevant authority and granted by the European Commission.
On 27 May 2021 the UK Health and Safety Executive submitted a reasoned request, under Article 55(1) of Regulation (EU) 528/2012, to extend the derogation for the use of biocidal hand sanitizer products in Northern Ireland. The two Implementing Decisions of 6 December 2021 reflect the Commission's positive response to the UK request – thus allowing for the continued use of the relevant hand sanitizer products in Northern Ireland during the pandemic. The Implementing Decisions have retroactive effect to cover the time period between the submission of the UK request and its acceptance by the EU.
To take another example, on 28 June 2021, Commission Implementing Regulation (EU) 2021/1064 was made with regard to Regulation EU 2016/429 – the ‘Animal Health Law’ mentioned earlier – and to several existing implementing regulations that exist under the ‘parent’ Animal Health Law (namely Commission Delegated Regulation (EU) 2019/2035, Commission Implementing Regulation (EU) 2021/520 and Commission Implementing Regulation (EU) 2020/1470). The purpose of the new Commission Implementing Regulation 2021/1064 is to make changes to the existing legislation so as to introduce specific codes (‘XI’ and ‘889’) to allow for the identification of certain kept terrestrial animals (e.g., cows, sheep, goats, deer etc.) and hatching eggs (e.g., chickens, ducks, turkeys, geese etc.) that originate in ‘the UK in respect of Northern Ireland’. Some minor changes are also made to clarify that the relevant legislation applies to Northern Ireland under the Withdrawal Agreement and Protocol.
Implementing legislation passed under EU acts that apply under the Protocol often does not relate to Northern Ireland. By way of example, on 12 November 2021 Commission Regulation (EU) 2021/1973 was passed to amend Regulation (EC) No 1069/2009 that lays down rules regarding animal by-products and derived products that are not intended for human consumption, and which applies to Northern Ireland under Article 5 and Annex 2 of the Protocol. The purpose of the new implementing Regulation (EU) 2021/1973 is to correct errors in the German language version of the ‘parent’ act Regulation (EC) No 1069/2009. So, while the new implementing legislation falls within the scope of the Protocol it has no policy significance for Northern Ireland. In another example, in 23 November 2021, Council Implementing Decision (EU) 2021/2058 was passed having regard to Council Directive 2003/96/EC that relates to the EU framework for taxation of energy products and electricity and which applies to Northern Ireland under Article 8 and Annex 3 of the Protocol. The purpose of this new Implementing Decision is to authorise Italy to apply a reduced rate of tax to any electricity supplied to maritime and inland waterway vessels, other than private pleasure crafts, that are at berth in an Italian port. So, again, while the new implementing legislation applies under the Protocol, its policy relevance to Northern Ireland is minimal.
Indeed, following the two previous examples, even when implementing legislation addresses Northern Ireland directly, the nature of the provisions made are likely to be very technical. The policy significance of changes at this level is, therefore, limited.
This is not to suggest that changes in relevant EU implementing legislation are unimportant for post-Brexit Northern Ireland; it is rather to put their significance in context. By definition, implementing legislation implements law that already applies. Changes under the Protocol arising from implementing legislation are therefore unlikely to have much impact in terms of substantive policy. Changes nevertheless do need to be tracked.
Tracking change, however, is not straightforward. New EU legislation is published in the Official Journal of the European Union, but determining which pieces of EU law apply to Northern Ireland, and which do not, requires detailed study and timely cross-referencing.
Currently, no comprehensive and publicly accessible register of all EU law applicable under the Protocol exists. The European Commission has committed to developing a website dedicated to providing a “clear and comprehensive” record of such legislation as part of its October 2022 package of measures for the implementation of the Protocol. At the time of writing, the website has not been launched.
One way of tracking the amount of relevant change is by looking at ‘consolidated text’ versions of applicable acts. When a substantial amount of implementing legislation has been made under an EU Directive, Regulation or Decision, the original legal text – the parent act – is often ‘recast’ as a ‘consolidated text’ to incorporate changes since the parent act was adopted.
While not a providing a comprehensive means of tracking change, this process of ‘recasting’ can be taken as an indicator of the extent of changes made to the application of a given EU act via implementing legislation.
Since the UK left the EU on 31 January 2020, almost a quarter of the EU acts that apply in Northern Ireland under the Protocol have been recast as consolidated texts. Of those 90 recast EU acts 62 were recast during 2021. These included, for example: Commission Regulation (EC) No 1295/2008 of 18 December 2008 on the importation of hops from third countries, consolidated on 1 January 2021; Regulation (EU) 2017/1369 of the European Parliament and of the Council of 4 July 2017 setting a framework for energy labelling and repealing Directive 2010/30/EU, consolidated on 1 May 2021; Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products, consolidated on 1 October 2021. A full list, including links to original and consolidated versions of the relevant EU acts, is provided in Appendix 1 to this explainer.
5. Research Database of Applicable EU Law
Pending the launch of a dedicated official resource on the EU acts applicable in post-Brexit Northern Ireland, the three-year ESRC-funded research project Governance for ‘a place between’: the Multilevel Dynamics of Implementing the Protocol on Ireland/Northern Ireland for which this explainer has been produced, has developed a freely accessible database that provides links to each applicable EU act as well as the relevant implementing acts adopted by the EU that can be found through the ‘consolidated text’ versions of the original acts. Through the database, the Project is recording additions, amendments, and replacements as well as the deletions and instances where, through expiry, an EU act no longer applies.
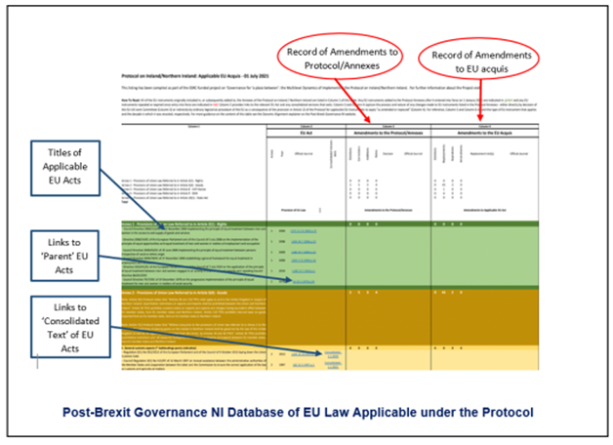
The database contains a list of all of those EU acts that apply in the UK ‘in respect of Northern Ireland’ through the Protocol as of 1 January 2022. It therefore reflects all of the ‘amendments and replacements’ to applicable EU law described in this explainer.
Conclusion: A dynamic democratic challenge
What is clear from this explainer is that implementation of the Protocol involves extensive legislative complexity.
Since the terms of the UK’s withdrawal from the EU were agreed in October 2019, the EU acquis that applies under the Protocol has changed. To date, however, the majority of the most substantive ‘amendments and replacements’ enact changes agreed while the UK was still part of the EU.
This reflects the often-slow pace of EU legislative processes and the fact that the EU acquis that applies in post-Brexit Northern Ireland – primarily concerning trade in goods – is relatively stable. This notwithstanding, ‘amendments or replacements’ made in the second year of the Protocol’s implementation, and thereafter, are much less likely to have originated while the UK was an EU member state.
This brings us back to the dual challenges of democratic accountability and legislative scrutiny that dynamic alignment and the maintenance of the conditions for the free movement of goods on the island of Ireland poses. These challenges follow from both the novelty of the Protocol’s provisions and the degree of transparency (or lack thereof) surrounding the activities of the three bodies set up to oversee it: the Joint Committee, Specialised Committee and Joint Consultative Working Group.
At present, there is limited provision for those directly impacted by Northern Ireland’s position under the Protocol to input into the process of dynamic regulatory alignment although mechanisms, such as the Joint Consultative Working Group, can provide some opportunity. Such mechanisms can also be used to convey the position of the UK government in respect of Northern Ireland on proposed EU legislation that the EU may seek to have applied – subject to the agreement of the UK in the Joint Committee – under the Protocol. The proposed EU Carbon-Border Adjustment Mechanism is a case in point.
Improving the level of engagement with those in Northern Ireland is one of the topics on the table in ongoing UK-EU talks about the implementation of the Protocol. Both the UK and the EU have proposed measures that would, respectively, provide a “stronger role” and “enhance engagement” with Northern Ireland stakeholders and authorities.
With the outcome of UK-EU talks still uncertain, the agreement in principle between the two sides that an enhanced role for those in Northern Ireland to whom EU laws continue to apply under the Protocol is welcome.
While post-Brexit dynamic alignment with the elements of the EU acquis applicable under the Protocol remains a contested issue in Northern Ireland, lack of provision for direct Northern Ireland input into the adoption of relevant EU acts adds more political tension to the already difficult task of implementing the Protocol. Whether the UK and EU can move from agreement in principle to actual enhanced Northern Ireland engagement on EU legislative developments relating to the Protocol remains to be seen.
January 2022
Download a copy of this Explainer here.
Dr. Lisa Claire Whitten is Research Fellow on the Governance for ‘a place between’: the multilevel dynamics of implementing the Protocol on Ireland/Northern Ireland at Queen's University Belfast. She can be contacted via: l.whitten@qub.ac.uk.
Appendix 1: EU Acts Applicable under the Protocol on Ireland/Northern Ireland and Published as ‘Consolidated Texts’ since UK Withdrawal from the EU on 31 January 2020
NB: Acts listed by date of consolidation up until and including 1 January 2022
|
|
EU Act |
Article Annex |
Official Journal |
Consolidated Text |
|
|
2020 |
|
|
|
|
1 |
Council Directive 68/193/EEC of 9 April 1968 on the marketing of material for the vegetative propagation of the vine |
Article 5 Annex 2 |
||
|
2 |
Council Directive 66/401/EEC of 14 June 1966 on the marketing of fodder plant seed |
Article 5 Annex 2 |
||
|
3 |
Council Directive 2002/56/EC of 13 June 2002 on the marketing of seed potatoes |
Article 5 Annex 2 |
||
|
4 |
Council Directive 2002/57/EC of 13 June 2002 on the marketing of seed of oil and fibre plants |
Article 5 Annex 2 |
||
|
5 |
Regulation (EU) No 1379/2013 of the European Parliament and of the Council of 11 December 2013 on the common organisation of the markets in fishery and aquaculture products amending Council Regulations (EC) No 1184/2006 and (EC) No 1224/2009 and repealing Council Regulation (EC) No 104/20001, insofar as it concerns provisions relating to marketing standards and consumer information |
Article 5 Annex 2 |
||
|
6 |
Directive (EU) 2016/797 of the European Parliament and of the Council of 11 May 2016 on the interoperability of the rail system within the European Union, insofar as conditions and technical specifications for the placing on the market, putting into service and free movement of railway products are concerned |
Article 5 Annex 2 |
||
|
7 |
Regulation (EU) 2020/740 of the European Parliament and of the Council of 25 May 2020 on the labelling of tyres with respect to fuel efficiency and other parameters, amending Regulation (EU) 2017/1369 and repealing Regulation (EC) No 1222/2009 |
Article 5 Annex 2 |
||
|
8 |
Council Directive 80/181/EEC of 20 December 1979 on the approximation of the laws of the Member States relating to units of measurement and on the repeal of Directive 71/354/EEC |
Article 5 Annex 2 |
||
|
9 |
Council Directive 2008/72/EC of 15 July 2008 on the marketing of vegetable propagating and planting material, other than seed |
Article 5 Annex 2 |
||
|
10 |
Council Directive 2002/55/EC of 13 June 2002 on the marketing of vegetable seed |
Article 5 Annex 2 |
||
|
11 |
Commission Regulation (EU) No 1407/2013 of 18 December 2013 on the application of Articles 107 and 108 of the Treaty on the Functioning of the European Union to de minimis aid |
Article 10 Annex 5 |
||
|
12 |
Regulation (EU) 2016/1036 of the European Parliament and of the Council of 8 June 2016 on protection against dumped imports from countries not members of the European Union |
Article 5 Annex 2 |
|
|
|
13 |
Regulation (EU) 2016/1037 of the European Parliament and of the Council of 8 June 2016 on protection against subsidised imports from countries not members of the European Union |
Article 5 Annex 2 |
|
|
|
14 |
Regulation (EU) 2016/1076 of the European Parliament and of the Council of 8 June 2016 applying the arrangements for products originating in certain states which are part of the African, Caribbean and Pacific (ACP) Group of States provided for in agreements establishing, or leading to the establishment of, economic partnership agreements |
Article 5 Annex 2 |
|
|
|
15 |
Regulation (EC) No 715/2007 of the European Parliament and of the Council of 20 June 2007 on type approval of motor vehicles with respect to emissions from light passenger and commercial vehicles (Euro 5 and Euro 6) and on access to vehicle repair and maintenance information |
Article 5 Annex 2 |
|
|
|
16 |
Regulation (EC) No 595/2009 of the European Parliament and of the Council of 18 June 2009 on type-approval of motor vehicles and engines with respect to emissions from heavy duty vehicles (Euro VI) and on access to vehicle repair and maintenance information and amending Regulation (EC) No 715/2007 and Directive 2007/46/EC and repealing Directives 80/1269/EEC, 2005/55/EC and 2005/78/EC |
Article 5 Annex 2 |
|
|
|
17 |
Regulation (EU) No 649/2012 of the European Parliament and of the Council of 4 July 2012 concerning the export and import of hazardous chemicals |
Article 5 Annex 2 |
||
|
18 |
Commission Regulation (EU) No 360/2012 of 25 April 2012 on the application of Articles 107 and 108 of the Treaty on the Functioning of the European Union to de minimis aid granted to undertakings providing services of general economic interest |
Article 10 Annex 5 |
||
|
19 |
Regulation (EU) No 168/2013 of the European Parliament and of the Council of 15 January 2013 on the approval and market surveillance of two- or three-wheel vehicles and quadricycles |
Article 5 Annex 2 |
|
|
|
20 |
Regulation (EU) 2017/821 of the European Parliament and of the Council of 17 May 2017 laying down supply chain due diligence obligations for Union importers of tin, tantalum and tungsten, their ores, and gold originating from conflict-affected and high-risk areas |
Article 5 Annex 2 |
|
|
|
21 |
Commission Regulation (EU) No 702/2014 of 25 June 2014 declaring certain categories of aid in the agricultural and forestry sectors and in rural areas compatible with the internal market in application of Articles 107 and 108 of the Treaty on the Functioning of the European Union |
Article 10 Annex 5 |
||
|
22 |
Commission Regulation (EU) No 1388/2014 of 16 December 2014 declaring certain categories of aid to undertakings active in the production, processing and marketing of fishery and aquaculture products compatible with the internal market in application of Articles 107 and 108 of the Treaty on the Functioning of the European Union |
Article 10 Annex 5 |
||
|
23 |
Commission Regulation (EU) No 717/2014 of 27 June 2014 on the application of Articles 107 and 108 of the Treaty on the Functioning of the European Union to de minimis aid in the fishery and aquaculture sector |
Article 10 Annex 5 |
||
|
24 |
Council Regulation (EC) No 1215/2009 of 30 November 2009 introducing exceptional trade measures for countries and territories participating in or linked to the European Union's Stabilisation and Association process (Western Balkans) |
Article 5 Annex 2 |
||
|
25 |
Chapter IV of Title V of Regulation (EU) No 1306/2013 of the European Parliament and of the Council of 17 December 2013 on the financing, management and monitoring of the common agricultural policy and repealing Council Regulations (EEC) No 352/78, (EC) No 165/94, (EC) No 2799/98, (EC) No 814/2000, (EC) No 1290/2005 and (EC) No 485/2008 |
Article 5 Annex 2 |
||
|
26 |
Section 1 of Chapter I of Title II of Part II of Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007 |
Article 5 Annex 2 |
||
|
27 |
Sections 2 and 3 of Chapter I of Title II of Part II of Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007 |
Article 5 Annex 2 |
||
|
28 |
Part III of Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/20073, with the exception of Chapter VI |
Article 5 Annex 2 |
||
|
|
2021 |
|
|
|
|
29 |
Council Regulation (EC) No 515/97 of 13 March 1997 on mutual assistance between the administrative authorities of the Member States and cooperation between the latter and the Commission to ensure the correct application of the law on customs and agricultural matters |
Article 5 Annex 2 |
|
|
|
30 |
Commission Regulation (EC) No 1295/2008 of 18 December 2008 on the importation of hops from third countries |
Article 5 Annex 2 |
||
|
31 |
Regulation (EU) 2019/125 of the European Parliament and of the Council of 16 January 2019 concerning trade in certain goods which could be used for capital punishment, torture or other cruel, inhuman, or degrading treatment or punishment |
Article 5 Annex 2 |
||
|
32 |
Council Regulation (EC) No 2368/2002 of 20 December 2002 implementing the Kimberley Process certification scheme for the international trade in rough diamonds |
Article 5 Annex 2 |
||
|
33 |
Directive 2003/87/EC of the European Parliament and of the Council of 13 October 2003 establishing a system for greenhouse gas emission allowance trading within the Union and amending Council Directive 96/61/EC |
Article 9 Annex 4 |
||
|
34 |
Regulation (EC) No 1013/2006 of the European Parliament and of the Council of 14 June 2006 on shipments of waste |
Article 5 Annex 2 |
||
|
35 |
Regulation (EC) No 273/2004 of the European Parliament and of the Council of 11 February 2004 on drug precursors |
Article 5 Annex 2 |
|
|
|
36 |
Council Regulation (EC) No 111/2005 laying down rules for the monitoring of trade between the Union and third countries in drug precursors |
Article 5 Annex 2 |
|
|
|
37 |
Regulation (EU, Euratom) No 883/2013 of the European Parliament and of the Council of 11 September 2013 concerning investigations conducted by the European Anti-Fraud Office (OLAF) and repealing Regulation (EC) No 1073/1999 of the European Parliament and of the Council and Council Regulation (Euratom) No 1074/1999 |
Article 5 Annex 2 |
|
|
|
38 |
Regulation (EU) No 654/2014 of the European Parliament and of the Council of 15 May 2014 concerning the exercise of the Union's rights for the application and enforcement of international trade rules and amending Council Regulation (EC) No 3286/94 laying down Community procedures in the field of the common commercial policy in order to ensure the exercise of the Community's rights under international trade rules, in particular those established under the auspices of the World Trade Organization |
Article 5 Annex 2 |
|
|
|
39 |
Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on persistent organic pollutants |
Article 5 Annex 2 |
||
|
40 |
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements |
Article 5 Annex 2 |
||
|
41 |
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs |
Article 5 Annex 2 |
||
|
42 |
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC |
Article 5 Annex 2 |
||
|
43 |
Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings |
Article 5 Annex 2 |
||
|
44 |
Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 |
Article 5 Annex 2 |
||
|
45 |
Regulation (EC) No 2065/2003 of the European Parliament and of the Council of 10 November 2003 on smoke flavourings used or intended for use in or on foods |
Article 5 Annex 2 |
||
|
46 |
Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC |
Article 5 Annex 2 |
||
|
47 |
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition |
Article 5 Annex 2 |
||
|
48 |
Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed, with the exception of the second paragraph of Article 32 |
Article 5 Annex 2 |
||
|
49 |
Part C of Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC |
Article 5 Annex 2 |
|
|
|
50 |
Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods |
Article 5 Annex 2 |
||
|
51 |
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health ("Animal Health Law") |
Article 5 Annex 2 |
||
|
52 |
Regulation (EC) No 2160/2003 of the European Parliament and of the Council of 17 November 2003 on the control of salmonella and other specified food-borne zoonotic agents |
Article 5 Annex 2 |
||
|
53 |
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’) |
Article 5 Annex 2 |
||
|
54 |
Regulation (EC) No 1760/2000 of the European Parliament and of the Council of 17 July 2000 establishing a system for the identification and registration of bovine animals and regarding the labelling of beef and beef products and repealing Council Regulation (EC) No 820/97 |
Article 5 Annex 2 |
||
|
55 |
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009 |
Article 5 Annex 2 |
||
|
56 |
Regulation (EU) 2017/1369 of the European Parliament and of the Council of 4 July 2017 setting a framework for energy labelling and repealing Directive 2010/30/EU |
Article 5 Annex 2 |
||
|
57 |
Directive 2009/48/EC of the European Parliament and of the Council of 18 June 2009 on the safety of toys |
Article 5 Annex 2 |
||
|
58 |
Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the definition, description, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No 1576/89 |
Article 5 Annex 2 |
||
|
59 |
Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use |
Article 5 Annex 2 |
||
|
60 |
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety |
Article 5 Annex 2 |
||
|
61 |
Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. |
Article 5 Annex 2 |
||
|
62 |
Regulation (EU) 2016/1628 of the European Parliament and of the Council of 14 September 2016 on requirements relating to gaseous and particulate pollutant emission limits and type-approval for internal combustion engines for non-road mobile machinery, amending Regulations (EU) No 1024/2012 and (EU) No 167/2013, and amending and repealing Directive 97/68/EC |
Article 5 Annex 2 |
|
|
|
63 |
Council Directive 2006/112/EC of 28 November 2006 on the common system of value added tax |
Article 8 Annex 3 |
||
|
64 |
Council Regulation (EU) No 904/2010 of 7 October 2010 on administrative cooperation and combating fraud in the field of value added tax |
Article 8 Annex 3 |
||
|
65 |
Council Directive 2009/132/EC of 19 October 2009 determining the scope of Article 143(b) and (c) of Directive 2006/112/EC as regards exemption from value added tax on the final importation of certain goods |
Article 8 Annex 3 |
||
|
66 |
Commission Delegated Regulation (EU) 2020/687 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and the Council, as regards rules for the prevention and control of certain listed diseases |
Article 5 Annex 2 |
|
|
|
67 |
Regulation (EC) No 765/2008 of the European Parliament and of the Council of 9 July 2008 setting out the requirements for accreditation and market surveillance relating to the marketing of products and repealing Regulation (EEC) No 339/93 |
Article 5 Annex 2 |
||
|
68 |
Regulation (EU) 2019/1241 of the European Parliament and of the Council of 20 June 2019 on the conservation of fisheries resources and the protection of marine ecosystems through technical measures, amending Council Regulations (EC) No 1967/2006, (EC) No 1224/2009 and Regulations (EU) No 1380/2013, (EU) 2016/1139, (EU) 2018/973, (EU) 2019/472 and (EU) 2019/1022 of the European Parliament and of the Council, and repealing Council Regulations (EC) No 894/97, (EC) No 850/98, (EC) No 2549/2000, (EC) No 254/2002, (EC) No 812/2004 and (EC) No 2187/2005 |
Article 5 Annex 2 |
||
|
69 |
Regulation (EU) No 305/2011 of the European Parliament and of the Council of 9 March 2011 laying down harmonised conditions for the marketing of construction products and repealing Council Directive 89/106/EEC |
Article 5 Annex 2 |
|
|
|
70 |
Directive 2004/42/EC of the European Parliament and of the Council of 21 April 2004 on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain paints and varnishes and vehicle refinishing products and amending Directive 1999/13/EC |
Article 5 Annex 2 |
||
|
71 |
Commission Regulation (EU) No 651/2014 of 17 June 2014 declaring certain categories of aid compatible with the internal market in application of Articles 107 and 108 of the Treaty |
Article 10 Annex 5 |
||
|
72 |
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives |
Article 5 Annex 2 |
||
|
73 |
Directive 2014/90/EU of the European Parliament and of the Council of 23 July 2014 on marine equipment and repealing Council Directive 96/98/EC |
Article 5 Annex 2 |
||
|
74 |
Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies |
Article 5 Annex 2 |
||
|
75 |
Regulation (EU) 2018/858 of the European Parliament and of the Council of 30 May 2018 on the approval and market surveillance of motor vehicles and their trailers, and of systems, components and separate technical units intended for such vehicles, amending Regulations (EC) No 715/2007 and (EC) No 595/2009 and repealing Directive 2007/46/EC |
Article 5 Annex 2 |
|
|
|
76 |
Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products |
Article 5 Annex 2 |
||
|
77 |
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC |
Article 5 Annex 2 |
||
|
78 |
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 |
Article 5 Annex 2 |
||
|
79 |
Council Regulation (EC) No 428/2009 of 5 May 2009 setting up a Community regime for the control of exports, transfer, brokering and transit of dual-use items |
Article 5 Annex 2 |
||
|
80 |
Directive 2009/43/EC of the European Parliament and of the Council of 6 May 2009 simplifying terms and conditions of transfers of defence-related products within the Community |
Article 5 Annex 2 |
||
|
81 |
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC |
Article 5 Annex 2 |
||
|
82 |
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin |
Article 5 Annex 2 |
||
|
83 |
Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation) |
Article 5 Annex 2 |
||
|
84 |
Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment |
Article 5 Annex 2 |
||
|
85 |
Commission Implementing Regulation (EU) No 543/2011 of 7 June 2011 laying down detailed rules for the application of Council Regulation (EC) No 1234/2007 in respect of the fruit and vegetables and processed fruit and vegetables sectors |
Article 5 Annex 2 |
||
|
86 |
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC |
Article 5 Annex 2 |
||
|
87 |
Regulation (EU) No 251/2014 of the European Parliament and of the Council of 26 February 2014 on the definition, description, presentation, labelling and the protection of geographical indications of aromatized wine products and repealing Council Regulation (EEC) No 1601/91 |
Article 5 Annex 2 |
||
|
|
2022 |
|
|
|
|
88 |
Regulation (EU) 2019/2152 of the European Parliament and of the Council of 27 November 2019 on European business statistics, repealing 10 legal acts in the field of business statistics |
Article 5 Annex 2 |
||
|
89 |
Regulation (EU) No 978/2012 of the European Parliament and of the Council of 25 October 2012 applying a scheme of generalised tariff preferences and repealing Council Regulation (EC) No 732/2008. |
Article 5 Annex 2 |
||
|
90 |
Council Directive 92/83/EEC of 19 October 1992 on the harmonization of the structures of excise duties on alcohol and alcoholic beverages |
Article 8 Annex 3 |
