COMPLEX I NEWS
Entire Complex I structure at 3.3 Å resolution is published
15 February 2013
Leonid Sazanov's lab fromCambridgereported the crystal structure of the entire Thermus thermophilus complex I at 3.3Å resolution (Baradaran et al., 2013, Nature). The structure suggests that a unique, out-of-the-membrane quinone-reaction chamber enables the redox energy to drive concerted long-range conformational changes in the four antiporter-like domains, resulting in translocation of four protons per catalytic cycle.
It was suggested that subunit NqoA (ND3 in mammalian enzyme) participates in the formation of the long and sealed quinone-reaction chamber. In mammalian complex I, a cysteine residue (Cys 39 ND3) equivalent to Ser46 of NqoA is accessible for chemical modification only in the “de-active” (D) form of the enzyme. In the published structure of bacterial enzyme, this serine is shielded from the solvent in the loop that provides part of the seal for the quinine chamber. It was suggested that in the de-active mammalian enzyme this loop changes the location which disrupts interaction of the quinone headgroup with its binding site and leading to loss of activity of the D-form.
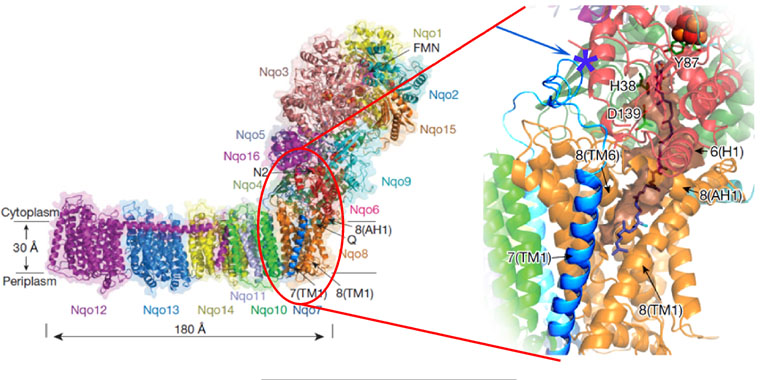
loop location ND3
Left: Structure of the entire complex I from T. thermophilus. Right: Theoretical model of bound ubiquinone-10. Subunit NqoA (ND3 in mammalian enzyme) is shown by bright blue. Nqo4 residues interacting with the quinine headgroup are indicated. Terminal cluster N2 is on the top. The quinone chamber is shown with surface in brown and helices framing its entry point are indicated. The location of Ser46 of NqoA (Cys-39 in ND3) is shown by blue arrow and asterisk (the detailed version of this figure can be found here: Baradaran et al., 2013, Nature)
Complex I membrane domain at 3.0 Å resolution has been released!
08 August 2011
Leonide Sazanov lab from Cambridge reported the crystal structure of the Esherichia coli complex I membrane domain at 3.0 Å resolution. It includes six subunits, NuoL, NuoM, NuoN, NuoA, NuoJ and NuoK, with 55 transmembrane helices. The fold of the homologous antiporter-like subunits L, M and N is novel, with two inverted structural repeats of five transmembrane helices arranged, unusually, face-to-back. Each repeat includes a discontinuous transmembrane helix and forms half of a channel across the membrane. A network of conserved polar residues connects the two half-channels, completing the proton translocation pathway. Unexpectedly, lysines rather than carboxylate residues act as the main elements of the proton pump in these subunits. The fourth probable proton-translocation channel is at the interface of subunits N, K, J and A. The structure indicates that proton translocation in complex I, uniquely, involves coordinated conformational changes in six symmetrical structural elements.
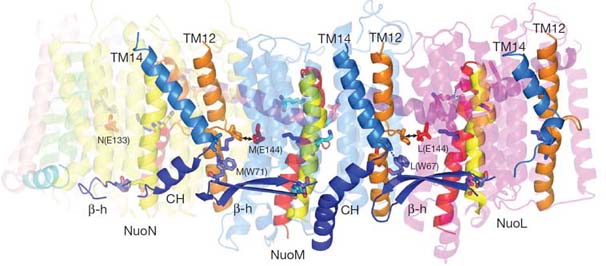
Connecting elements and interactions between subunits of the membrane domain of complex I from E. coli.
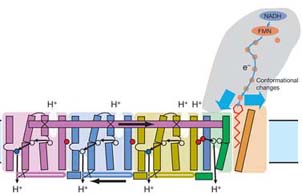
Conformational coupling between electron transfer and proton translocation is mediated by helix HL (cytoplasmic side) and the βH element (periplasmic side). A four protons per cycle are translocated per one molecule of NADH oxidised
Based on Complex I structure, the proton-translocating machinery of the enzyme provides stoichiometry of 4 H+/2 e- as has been directly measured in intact mitochondria, bovine SMP and Y. lipolytica Complex I-containing proteoliposomes [Wikstrom, 1984; Galkin et al., 1999; Galkin et al., 2006]. It makes respiratory Complex I is the major contributor to generation of protonmotive force across the inner mitochondrial membrane and one of the most intricate architectures among known protein structures.
Rouslan G. Efremov & Leonid A. Sazanov Structure of the membrane domain of respiratory complex I, Nature, (2011) DOI:10.1038/nature10330
1 July 2010
Uli Brandt's lab from Frankfurt reported an x-ray crystallographic analysis of mitochondrial complex I from Y. lypolytica. The domain structure supports the idea of conformational coupling of electron transfer with proton pumping and excludes direct mechanisms (i.e. Mitchell’s proton loop).
Hunte C, Zickermann V, BrandtU.2010, Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science, 329, 448-451. DOI: 10.1126/science.1191046

NEWS: Complex I structure has been solved!
The structures of the entire complex I from Thermus thermophilus have been determined in the group of Leo Sazanov in Cambridge. Complex I was the last component of the respiratory chain for which complete structure was unknown. The structures provide important insights about the mechanism of electron-proton coupling.
Rouslan G. Efremov, Rozbeh Baradaran & Leonid A. Sazanov, 2010, The architecture of respiratory complex I, Nature, 465, 441–445, DOI: doi:10.1038/nature09066
